Jared Lopez v. Nancy L. Newman: $12K Dog Bite Verdict
On Christmas night in 2022, a holiday gathering in Middletown, Connecticut, turned into a legal battle after a pit bull mix bit a guest in the face. Jared Lopez alleged that while he sat in the living room at 11:30 p.m., Nancy L. Newman brought the dog up from the basement, at which point the animal jumped onto his lap and attacked. The resulting injuries included permanent facial scarring and a loss of sensation in the lip. On January 21, 2026, a jury found Newman strictly liable under state la...
Read MoreRecent Articles
Are You an Expert Witness?
Increase your visibility and get more cases
Top Areas of Law
Latest Verdicts & Settlements

On Christmas night in 2022, a holiday gathering in Middletown, Connecticut, turned into a legal battle after a pit bull mix bit a guest in the face. Jared Lopez alleged that while he sat in the living room at 11:30 p.m., Nancy L. Newman brought the dog up from the basement, at which point the animal jumped onto his lap and attacked. The resulting injuries included permanent facial scarring and a loss of sensation in the lip. On January 21, 2026, a jury found Newman strictly liable under state law and awarded Lopez $12,831.50 in damages.
 Sohini C.
Sohini C.
In May 2020, Michaela Nielsen underwent a vaginal delivery at HCA Florida Kendall Hospital under the care of Dr. Eric Runyon. Following the birth, Ms. Nielsen alleged that she suffered a significant clitoral and labial laceration that the medical team failed to diagnose or repair. The Plaintiff contended that this oversight led to physical disfigurement and required multiple corrective surgeries. However, the defense maintained that Dr. Runyon provided appropriate care and that the injury was not a result of medical negligence. After a multi-day trial in the 11th Judicial Circuit Court of Florida, the jury returned a complete defense verdict, finding that Dr. Runyon was not negligent in his treatment of the Plaintiff.
 Sohini C.
Sohini C.
The legal battle between International Firearms South, Inc. and Glatze Militum, LLC concluded in a Miami-Dade courtroom after years of allegations involving missing funds and broken promises. International Firearms launched the lawsuit claiming that Yevgeniya Carita, acting for Glatze, had siphoned $400,000 from their joint venture bank accounts without permission. The plaintiff argued this was a clear case of conversion and a breach of fiduciary duty that crippled their business operations. However, the defense maintained that the plaintiff had failed to meet its own contractual obligations. In a sweeping decision, the jury found that International Firearms had not performed its essential duties under the agreement, nor was it excused from doing so. Consequently, the jury cleared the defendants of all charges, and the court ruled that the plaintiff would recover nothing from the suit.
 Sohini C.
Sohini C.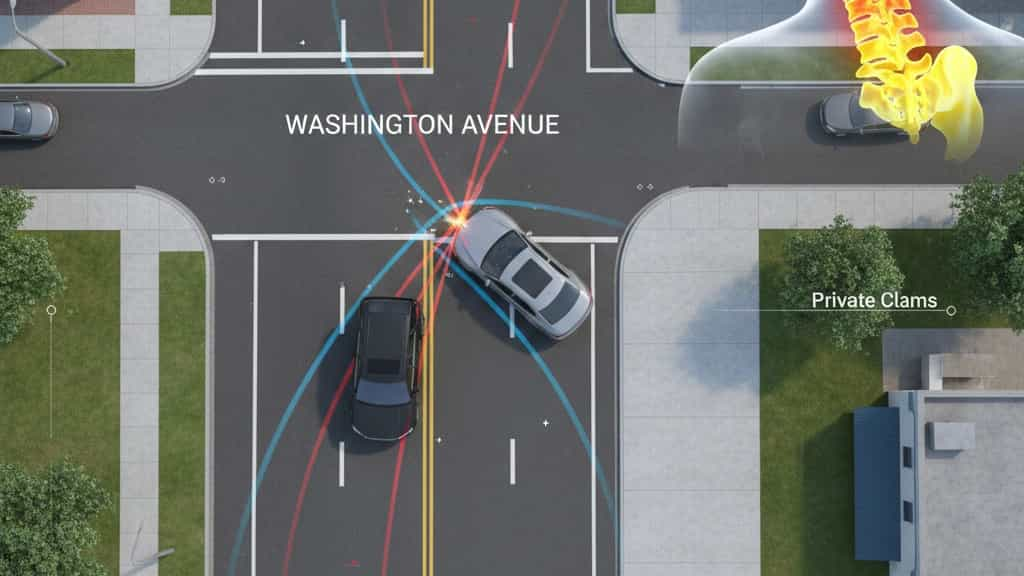
In the matter of James Beers v. Tyler Peruta et al., the Connecticut Superior Court addressed a significant motor vehicle negligence claim stemming from an October 26, 2021, collision. The plaintiff, James Beers, alleged that Tyler Peruta negligently exited a private driveway at 319 Washington Avenue in North Haven, turning left into the path of his southbound vehicle. Beers reported a comprehensive list of injuries, including cervical disc protrusions, retrolisthesis, and chronic radiculopathy, which he claimed led to permanent physical impairment and lost wages. While the defense admitted that the vehicle was operated with the owner Donald Peruta's authorization, they staunchly denied all allegations of negligence. Following a trial before the Honorable LaTonia Williams, the jury returned a verdict on September 12, 2025, in favor of the defendants. The court entered a final judgment of no liability, resulting in no monetary recovery for the plaintiff.
 Sohini C.
Sohini C.
Granby Tenant Loses Lawsuit Over Defective Handrail Fall
March 3, 2026
Carol Cruz, a tenant at 101 Meadow Gate Road in Granby, Connecticut, filed a lawsuit against her landlord, Halmar, Incorporated, following a traumatic fall on December 26, 2021. Cruz alleged that as she descended the interior stairs at approximately 11:45 p.m., she slipped and fell due to a non-compliant and dangerous handrail. The complaint detailed specific violations of state building and fire safety codes, claiming the 2 X 6 deck railing used as a handrail was too large to grasp and was positioned several inches lower than legally required. As a result of the tumble, Cruz suffered a fractured right hip and sought damages for medical expenses and permanent physical impairment. Halmar, Incorporated countered with a special defense, asserting that Cruz’s own negligence and failure to maintain a proper lookout caused the accident. On February 13, 2026, a jury in the Hartford Superior Court delivered a verdict in favor of the Defendant, Halmar, Incorporated, awarding no damages to the Plaintiff.
 Sohini C.
Sohini C.
On November 28, 2022, Vinod Singal sustained injuries after falling over an unmarked single step at the Imperial House Condominium in Miami-Dade County. Singal alleged the transition from the lobby to the exit was camouflaged and lacked the yellow safety markings present on other steps throughout the property. Claiming significant shoulder and rib injuries, Singal sought over $50,000 in damages for medical bills and pain and suffering. The defense argued the step was an "open and obvious" condition and that Singal’s own negligence caused the accident. After a three-day trial in October 2025, the jury found no negligence on the part of the Defendant, resulting in a final judgment where the Plaintiff received nothing.
 Sohini C.
Sohini C.
For years, the Gran Logia de Cuba A.L. & A.M., Inc. served as a pillar for Miami’s Masonic community, but a 2019 decision to modernize the organization sparked a civil war. After a supermajority of members voted to join the "regular" Florida Masons, two rival factions—the Camejo Group and the Tandron Group—claimed total control of the lodge’s heritage, bank accounts, and its physical headquarters on NW 22nd Avenue. The Tandron Group seized the building and changed the locks, leading to a high-stakes legal battle over corporate authority. Following a five-day trial, a Miami-Dade jury determined that the 2019 vote was valid and that the Camejo Group was the legitimate Board of Directors. While the jury awarded only nominal damages for trespass, the verdict effectively ended the "rogue" takeover, restoring the lodge to the leaders chosen by its members and ensuring the organization’s future under the Grand Lodge of Florida.
 Sohini C.
Sohini C.
The legal battle stemmed from an April 2021 traffic accident at the intersection of South Tryon and Stonewall Streets in Charlotte, North Carolina. Jacqueline Smith was driving her 2010 Audi through a green light when Steven Demond Springs, driving a 2014 Volkswagen, ran a red light and struck her vehicle. Ms. Smith sustained physical injuries and incurred significant medical expenses due to the impact. Following the death of Mr. Springs during the litigation, Mary Immen was substituted as the defendant in her capacity as the Administrator of his estate. Despite defense arguments suggesting contributory negligence, a Mecklenburg County jury found the defendant liable, awarding Ms. Smith $5,500 in damages for her personal injuries.
 Sohini C.
Sohini C.
Ex-De Tomaso CEO Gets $540K, Owes $129K in Verdict
March 2, 2026
The storied revival of De Tomaso Automobili culminated in a complex legal showdown in the New York Southern District Court. Ryan Berris, the former CEO and Chief Marketing Officer, alleged he was the architect of the brand's rebirth, only to be ousted by Norman Choi amidst disputes over a SPAC merger and "unethical" business practices. While Berris sought millions for lost equity and defamation, the jury's February 5, 2026, verdict painted a nuanced picture of the partnership. The jury rejected Berris’s breach of contract and defamation claims but awarded him $540,502.36 under the theory of quantum meruit for services rendered. Simultaneously, the jury found Berris liable for breaching his fiduciary duties of loyalty and care to the company, awarding De Tomaso $129,069.36 in damages. This verdict marks the end of a bitter transition for the legendary Italian marque, balancing unpaid compensation against executive misconduct.
 Sohini C.
Sohini C.
Riggins v. Walmart: $1.7M Slip and Fall Verdict Analysis
February 27, 2026
In the case of Riggins, Deloris vs. Finch, David et al. (Case No. 2021-CA-008187-O), the Ninth Judicial Circuit Court of Florida addressed a significant premises liability claim stemming from an incident at a Walmart Neighborhood Market in Orlando. On August 4, 2020, the Plaintiff, Deloris Riggins, tripped over a floor mat in the produce section, resulting in permanent bodily injuries and the aggravation of pre-existing conditions. The litigation centered on whether Walmart and its store manager breached their duty of care by failing to secure the rug or warn customers of the hazard. While the defense argued the danger was "open and obvious" and attributed the injuries to pre-existing health issues, the jury reached a unanimous verdict on September 19, 2025. Although the jury calculated total damages at $1,698,000—including $1.2 million for future medical expenses—they apportioned 70% of the fault to the Plaintiff under Florida’s comparative negligence rules. Consequently, the final judgment ordered Walmart Stores East, LP to pay a reduced sum of $509,400, highlighting the critical impact of fault apportionment in personal injury litigation.
 Sohini C.
Sohini C.
Duncan v. CT Transit: Jury Rules on New Haven Bus Accident
February 27, 2026
On August 3, 2022, Sherlette Duncan traveled as a passenger on a city bus heading northbound on Broadway Street in New Haven. The trip took a violent turn when a BMW, operated by Chiara Corazzini, collided with the bus while attempting to parallel park. Duncan alleged that the impact caused her body to jerk and twist, resulting in debilitating injuries to her lower back and legs, including sciatica and a misalignment of her spinal discs. Duncan subsequently filed a lawsuit against the Connecticut Transit District Consortium and the owners of the BMW, claiming the bus driver failed to maintain a safe lookout and that the transit authority breached its duty to provide the highest degree of care to its passengers. The defense countered by questioning the specific involvement of the transit district and the extent of the negligence alleged. After a full trial in the New Haven Superior Court, the jury found in favor of the Defendants, concluding that the Plaintiff was not entitled to the monetary damages she sought for her medical expenses and pain and suffering.
 Sohini C.
Sohini C.
Fróes v. ROC Funding: $1.5M Verdict for Predatory Lending
February 26, 2026
In a significant victory for small business owners, a California jury awarded Stella Ferreira Fróes, owner of Pikanhas Restaurant Group, over $1.5 million in damages against ROC Funding Group, LLC. The case (MSC21-01827) centered on a "loan consolidation" agreement that carried a hidden 69.9% interest rate. Despite Fróes’s compliance, ROC utilized a "Judgment by Confession" in New York to freeze her accounts and shutter her operations. The jury found ROC liable for usury and abuse of process, awarding $1.125 million in punitive damages to penalize the company's malicious and fraudulent conduct.
 Sohini C.
Sohini C.On the Stand with Ashish Arun
A podcast on the law, practice and business of expert witness testimony. Listen to the latest episodes.